Introduction: Patients with advanced cardiac AL have high early mortality despite receiving effective plasma cell-directed therapies. Cardiac dysfunction is caused by direct myocardial toxicity from amyloidogenic light chains (LCs) and architectural distortion from amyloid fibril deposition. Diagnosis before significant fibril-induced cardiac dysfunction is critical for improving outcomes. While measuring cardiac biomarkers is standard for risk stratification of patients with newly diagnosed AL, serum concentrations can be impacted by fluid shifts and renal impairment, suggesting a need for complementary biomarkers. Our transcriptomic analysis highlighted dysregulation of myocardial contractility in cardiac cells exposed to amyloidogenic LCs, leading us to hypothesize that there could be clinically measurable differences in cardiac contractility (Ghosh et al., 2023). Global longitudinal strain (GLS) is a sensitive echocardiographic measure of myocardial contractility and may predict survival independent of serum cardiac biomarkers. Since LC-induced cardiac dysfunction via direct myocardial toxic effects is reversible with plasma cell-directed therapy and may represent an early cardiac insult in patients with cardiac AL, we identified and evaluated patients with LC-induced cardiac dysfunction and no clinical or echocardiographic evidence of significant cardiac amyloid fibril deposition to determine clinical features potentially associated with early cardiac AL.
Methods: We analyzed the demographic, clinical, and echocardiographic features of patients with non-cardiac biopsy-proven AL amyloidosis and LC-induced cardiac dysfunction seen at the Boston University Amyloidosis Center between January 1, 2011 and March 31, 2023. Direct LC-induced cardiac dysfunction was defined as: (1) elevated cardiac biomarkers (BNP >176 pg/mL or NT-proBNP >899 pg/mL or troponin I >0.033 ng/mL, per institutional reference ranges); (2) normal echocardiographic interventricular septal thickness (<11 mm in males and <10 mm in females); (3) estimated glomerular filtration rate >60 mL/min/1.73m 2; (4) absence of late gadolinium enhancement on cardiac magnetic resonance imaging (if performed); and (5) negative endomyocardial biopsy (if performed). Echocardiographic variables, including left ventricular ejection fraction (LVEF) and GLS, were collected from the electronic health record or post-processed manually using TomTec 2D CPA. All statistical analyses were performed using GraphPad Prism version 8©. Overall survival (OS) was evaluated using the Kaplan-Meier estimator and log rank tests.
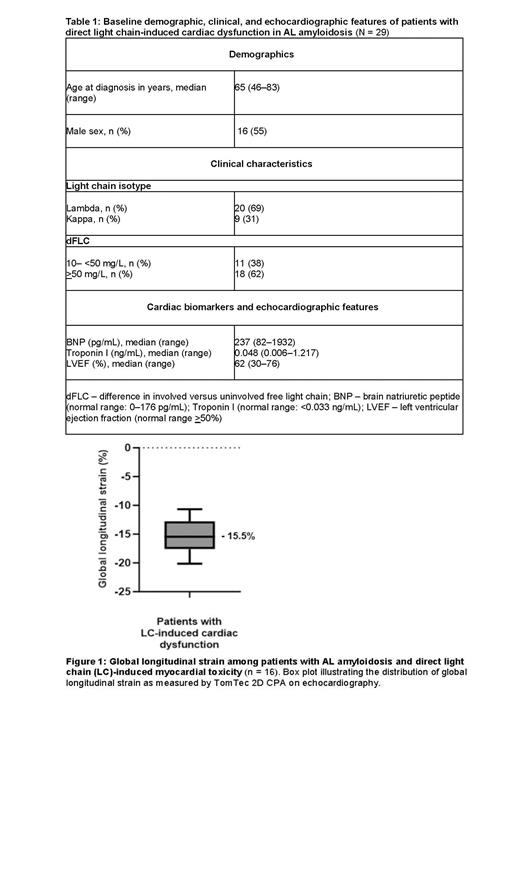
Results: Twenty-nine patients met our criteria for direct LC-induced cardiac dysfunction. Median age at diagnosis was 65 years (range: 46-83), and 55% of patients (n=16) were male ( Table 1). LC isotype was lambda in 69% (n=20) and kappa in 31% (n=9) of patients. The difference in free light chains (dFLC) was >50 mg/L in 62% (n=18) of patients at diagnosis.Additionally, 76% (n=22) of patients met criteria for Boston University stage II cardiac disease with mildly elevated cardiac biomarkers (median BNP 237 pg/mL, median troponin I 0.053 ng/mL). Among 16 patients for whom a GLS measurement was available, 81% (13/16) had an abnormal GLS (normal < -18%). Median and mean GLS were -15.5% (-7 to -21.7) and -15.3% ± 4.5, respectively ( Figure 1). Median OS was 5.2 years for the entire cohort.
Conclusion: We found that a subset of patients with AL amyloidosis demonstrate cardiac dysfunction without substantial clinical or echocardiographic evidence of amyloid deposition; this we postulate to be the result of direct myocardial toxic effects by amyloidogenic LCs. Early cardiac biomarker stage and abnormal GLS were commonly associated with LC-induced cardiac dysfunctionin these patients.
Disclosures
Sanchorawala:Janssen, Alexion, Prothena, Celgene, Takeda, Abbvie, Regeneron, Pfizer, AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.